GFP-Trap Affinity Reagents
Novel Antibody Fragment that binds GFP Fusion Proteins
Analyze GFP fusion proteins – in a tube
Fluorescent fusion proteins are popular tools to study protein localization and cellular dynamics. These constructs usually fuse the entirety–or at least a functional domain–of a target protein to one of the many types of reporter fluorescent proteins. Those originally derived from the jellyfish A. victoria are referred to as green fluorescent protein, or GFP. It’s easy to image cells using a GFP-fusion protein construct, though to get the full picture such imaging data is often combined with additional biochemical information for the protein (or protein domain) of interest. For such biochemical experiments, typically a second fusion protein is designed using a different “tag” domain that allows for purification. These additional in vitro analyses can be used to confirm functionality of the “tagged” fusion construct, as well as to pull out multi-protein complexes that may form in the cellular milieu. The lack of specific, reliable, and efficient reagents has limited the use of GFP, and related fluorescent fusion proteins, for use in both cell biology studies and direct biochemical analysis.
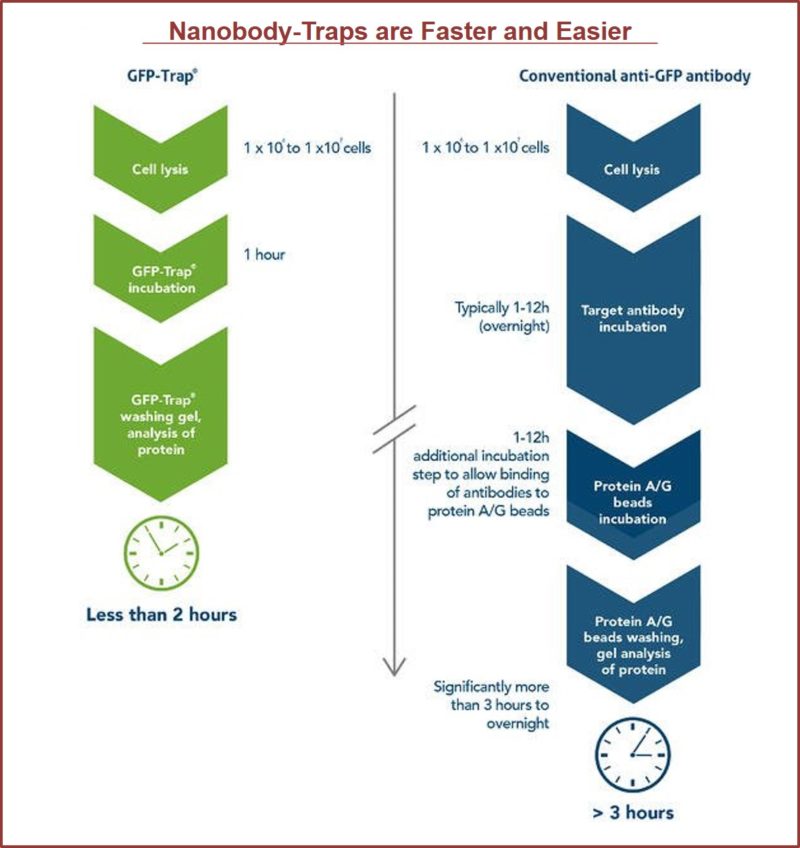
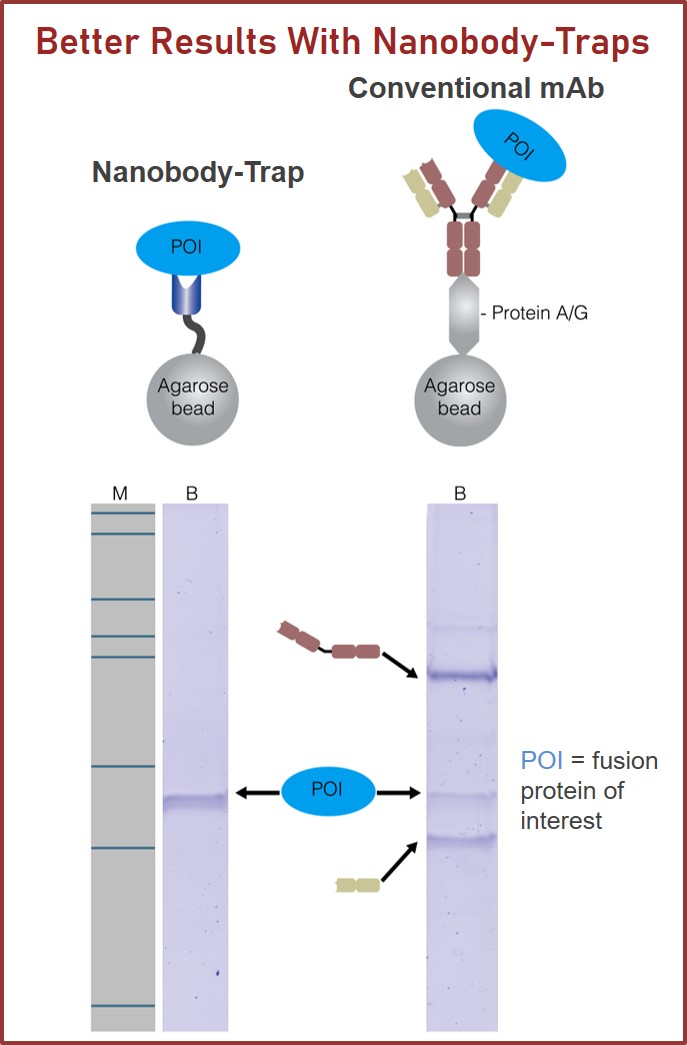
GFP-Traps utilize super-high affinity alpaca antibody fragments coupled to agarose beads or magnetic agarose beads. These “Nanobody-Traps” are perfect for immuno-precipitation, immuno-purification, and immuno-pulldown experiments with up to 10-fold better purity (and yield) than that of conventional mouse monoclonal antibodies. Compatible with a variety of source materials, Nanobody-Traps may be used with mammalian cells, tissues & organs, bacteria, yeast, and even plants. These reagents allow your GFP-fusions to be perfect candidates for immunoprecipitations, Co-IP, mass spectroscopy, enzyme activity measurements, and ChIP analysis.

GFP-Trap Affinity Reagents are perfect for fast, clean, and efficient one-step immuno-purification of these fluorescent fusion proteins and their interacting factors. Manufactured since 2008, and with thousands of published citations attesting to their broad use and effectiveness, we invite you to try this unique reagent for free. They’ve become a staple of cell biology research throughout Europe and North America.
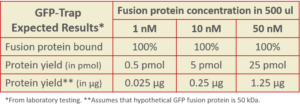
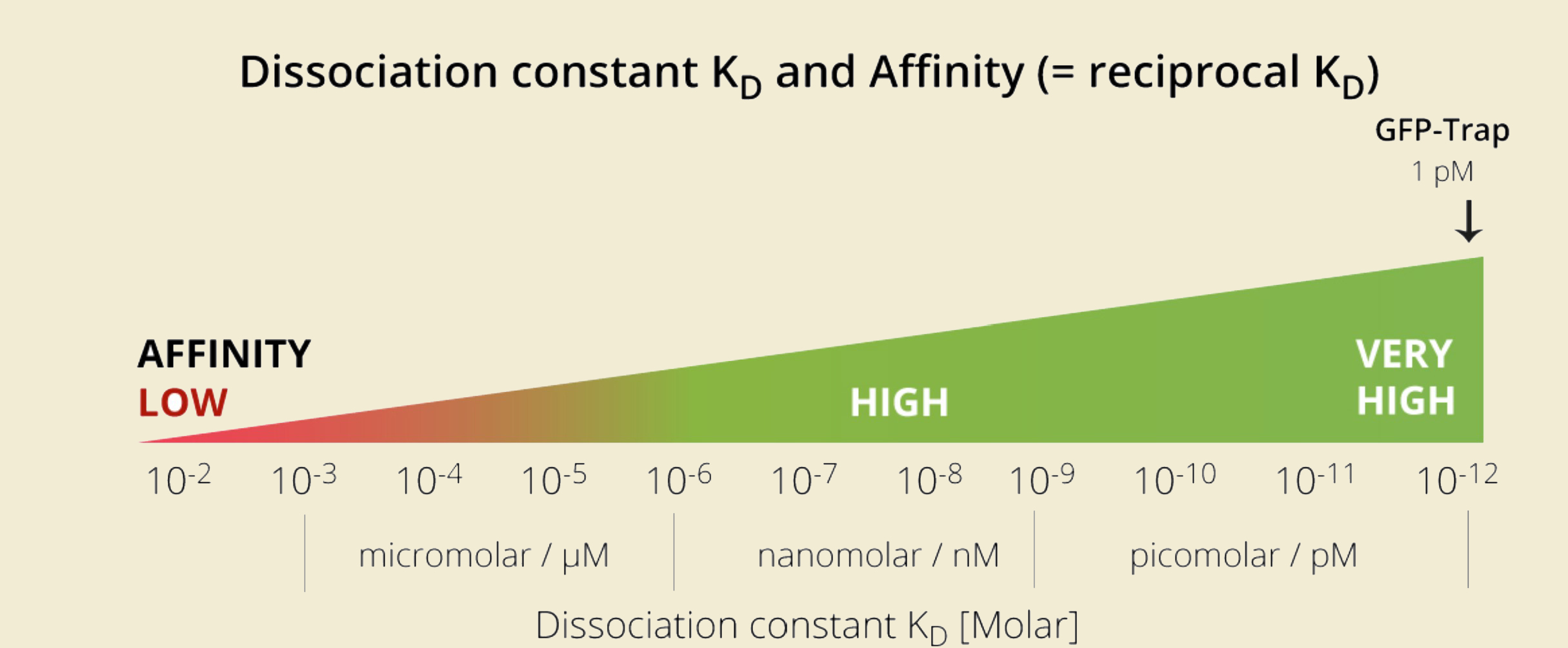
GFP-Trap has affinity significantly higher than most antibodies and a phenomenal Kd over a range of fusion protein concentrations!
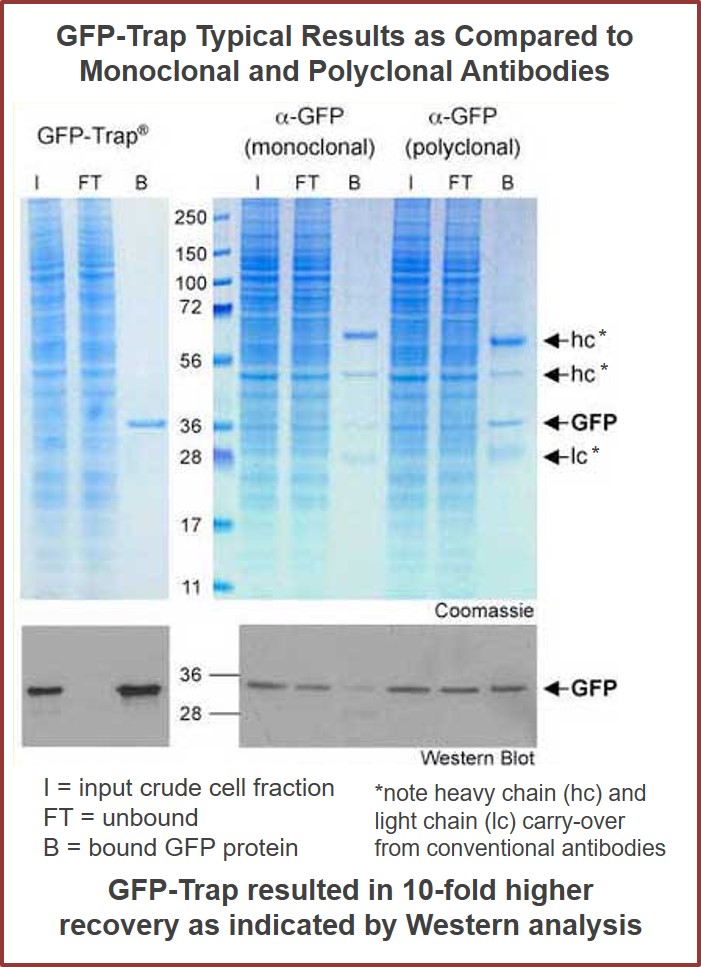
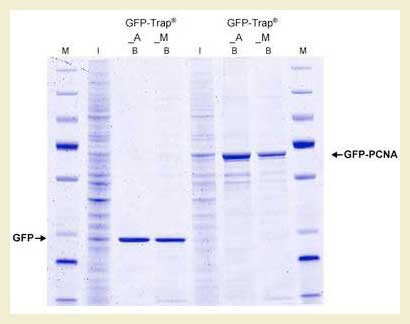
Protein complexes can’t hide from Nanobody-Traps
To the right are results from using GFP-Trap bound to magnetic particles (GFP-Trap M) and agarose beads (GFP-Trap A). Comparison of GFP-Trap A and GFP-Trap M was first performed with a simple, 1-step pull-down of native GFP with GFP-Trap A and GFP-Trap M from 293T cell extracts. Input (I) and bound (B) fractions were separated by SDS-PAGE followed by Coomassie staining. As expected, the bound fractions containing GFP are extraordinarily pure. The same 1-step purification procedure was then applied to GFP fused to the protein PCNA in a co-immunoprecipitation experiment. This time, the chimera and any associated proteins were very efficiently purified with both GFP-Trap A and GFP-Trap M from 293T cell extracts. As can be seen from the gel, the additional bands represent potential binding partners for the GFP-PCNA.
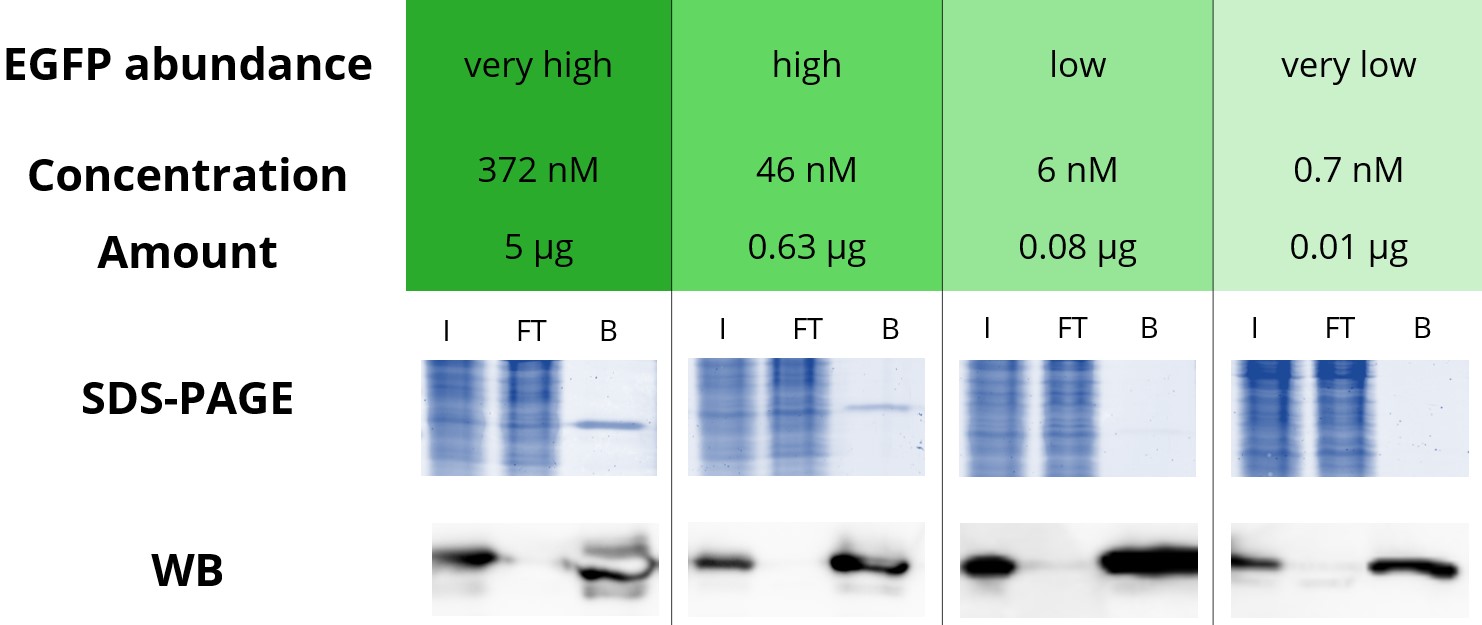
Immunoprecipitation (IP) of proteins expressed at low levels are no match for GFP-Trap
Below is an immunoprecipitation of various concentrations of EGFP with GFP-Trap Agarose.
4 defined amounts of EGFP were spiked into 500 µL diluted HEK293T cell lysate. IP was performed according to the GFP-Trap manual and bound EGFP was eluted with 2x SDS-sample buffer. Input (I), flow through (FT), and bound (B) fractions were analyzed by SDS-PAGE and Western blot (WB).
What’s your lab’s favorite flavor?
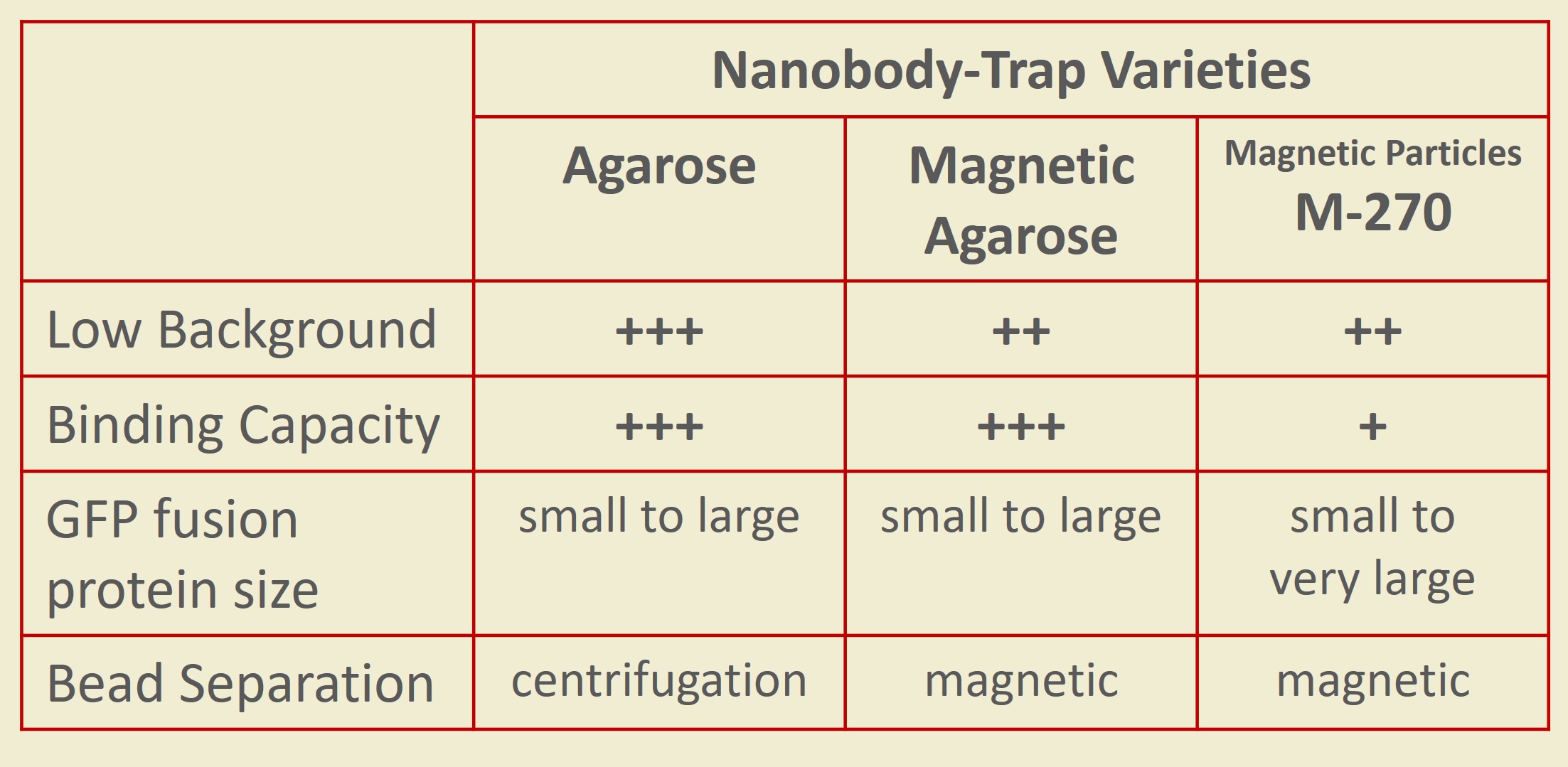
Nanobody-Traps are high quality binding proteins coupled to a monovalent matrix (agarose beads, magnetic particles) for biochemical analysis of many fusion proteins and their interacting partners. The different formats allow you to perform a multitude of experiments. The most popular forms are Nanobody-Traps linked to agarose beads. Agarose beads provide the largest binding capacity and lowest background, while still being easy to work with. If automation is important to your lab, then magnetic agarose particles are a great choice. The purified protein complexes are compatible with down-stream analyses such as mass spectroscopy.
Over 50 fluorescent proteins have been checked with Nanobody-Traps, click to check!
Camelidae single-domain antibodies are like IgGs on steroids
The family of animals known as Camelidae (camels, llamas, and alpacas) produce functional antibodies devoid of light chains, so-called “heavy chain” antibodies. These heavy chain antibodies recognize and bind their antigens via a single variable domain. When cleaved from their carboxy tail, these barrel-shaped structures (2×3 nm) are extraordinarily small, naturally-occurring, and intact antigen binding fragments (MW of 13 kDa). These fragments, called Nanobodies, are characterized by high specificity, affinities in the low nanomolar range, and dissociation constants in the sub-nanomolar range (typically 10- to 100-fold better than mouse IgGs). The compact size of Nanobodies makes them extremely stable at temperatures up to 70°C, and functional even in 2M NaCl or 0.5% SDS. These small and powerful antibody fragments can be used in a variety of unique applications. They will open up your research possibilities.
Additional Information
“The GFP-Trap worked great, with nice robust pull-down of SEP-tagged protein!”
Alisa, Colorado State University 2019
“The beads worked great. I used them to pull down a nuclear protein over expressed tagged with GFP in hek293t. The protocol worked great and had a good Western blot from the eluted fractions.”
David, Baylor University 2018
“Yes we received the sample. I’ve got to say, I was pleased with the results. We used an adenovirus to express gfp and a gfp tagged protein and compared the gfp-trap beads to a traditional approach with magnetic dynabeads and an antigfp monoclonal antibody.”
Jared, University of Iowa 2017
GFP-Traps Coupled to Agarose

GTA010 – GFP-Trap, coupled to agarose, 10 rxns, 0.25 ml
Original price was: $289.00.$284.00Current price is: $284.00. Add to cart
GTA020 – GFP-Trap, coupled to agarose, 20 rxns, 0.5 ml
Original price was: $499.00.$489.00Current price is: $489.00. Add to cartGFP-Traps Coupled to Magnetic Agarose Beads

GTMA010 – GFP-Trap, coupled to magnetic agarose beads, 10 rxns, 0.25 ml
Original price was: $289.00.$284.00Current price is: $284.00. Add to cart
GTMA020 – GFP-Trap, coupled to magnetic agarose beads, 20 rxns, 0.5 ml
Original price was: $499.00.$489.00Current price is: $489.00. Add to cartGFP-Traps Non-Coupled
Binding Controls
Protein Controls
Spin Columns
|
SPECIFICATIONS
|
|
|---|---|
| Configuration: | |
|
GFP-Trap A |
Specific Camelidae antibody linked to agarose bead |
|
Part Numbers
|
GTA010, GTA020, GTA100, GTA200, GTA400, GTAK020, SCT20, SCT50 |
|
GFP-Trap MA |
Specific Camelidae antibody linked to magnetic agarose particle |
|
Part Numbers
|
GTMA010, GTMA020, GTMA100, GTMA200, GTA400, GTMAK020 |
|
GFP-Trap |
Uncoupled and purified Camelidae antibody |
|
Part Numbers
|
EGFP250, GT250, GTB250 |
| Specificity: | |
|
GFP-Traps |
GFP, eGFP, wtGFP, GFP S65T, TagGFP, AcGFP, eYFP, YFP, Venus, Citrine, eCitrine, CFP
(does not recognize TurboGFP or all RFPs) |
| Particle Size: | |
|
GFP-Trap A |
~90 µm |
|
GFP-Trap MA |
~40 µm |
|
GFP-Trap |
No particle coupled |
| Storage Buffer: | |
|
GFP-Trap A |
20% EtOH |
|
GFP-Trap MA |
20% EtOH |
|
GFP-Trap |
1x PBS; Preservative: 0.01% Sodium Azide |
| Storage and Stability: | |
|
GFP-Trap A |
store at 4°C; stable for one year. Do not freeze. |
|
GFP-Trap MA |
store at 4°C; stable for one year. Do not freeze. |
|
GFP-Trap |
store at 4°C; stable for one year. Do not freeze. |