Myc-Trap Affinity Reagents
Capture Myc-tagged Proteins
Purify Myc-tagged proteins without contaminating peptides
The Myc-Trap is a single chain polypeptide antibody coupled to agarose beads or magnetic agarose beads for immunoprecipitation of Myc-tagged proteins. All Nanobody-Traps use a recombinant antibody fragment lacking a heavy chain. For this reason the Myc-Trap cannot dissociate from the agarose bead carrier, and therefore offers the cleanest pulldown results. The Myc-Trap recognizes the Myc-tag sequence EQKLISEEDL at the N-terminus, C-terminus, or internal site of any fusion protein. We guarantee you’ll see the fastest and most efficient performance! Typical results yield much lower background than other commercial or home-made affinity reagents. This makes Myc-Trap perfect for labs who need crystal-clean results for applications like mass spectrometry or other persnickety analyses.
Better than traditional monoclonal antibodies
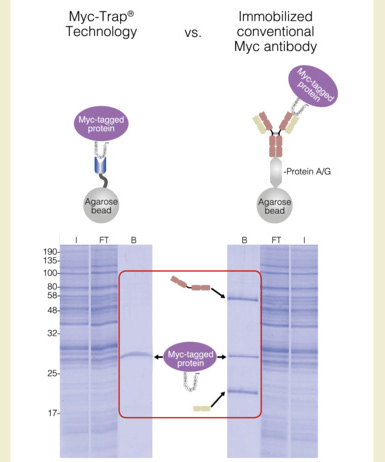
To the right is a pull-down experiment comparing the Myc-Trap technology to a conventional monoclonal Myc antibody. Most researchers today use the immobilized anti-Myc antibody 9E10 for immunoprecipitation of Myc fusion-proteins. During immunoprecipitation with 9E10, heavy and light antibody chains can dissociate from the immobilized 9E10 and contaminate the eluate of the Myc-tagged protein of interest with these antibody molecules. As you can see from the data, the single polypeptide chain used in the Myc-Trap does not suffer from this issue and provides clean results without contaminating peptides.
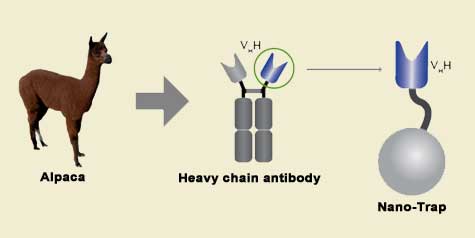
Camelidae single-domain antibodies are like IgGs on steroids
The family of animals known as Camelidae (camels, llamas, and alpacas) produce functional antibodies devoid of light chains, so-called "heavy chain" antibodies. These heavy chain antibodies recognize and bind their antigens via a single variable domain. When cleaved from their carboxy tail, these barrel-shaped structures (2x3 nm) are extraordinarily small, naturally-occurring, and intact antigen binding fragments (MW of 13 kDa). These fragments, called Nanobodies, are characterized by high specificity, affinities in the low nanomolar range, and dissociation constants in the sub-nanomolar range (typically 10- to 100-fold better than mouse IgGs). The compact size of Nanobodies makes them extremely stable at temperatures up to 70°C, and functional even in 2M NaCl or 0.5% SDS. These small and powerful antibody fragments can be used in a variety of unique applications. They will open up your research possibilities.
MycTraps Coupled to Agarose
Myc-Traps Coupled to Magnetic Agarose Beads
Binding Controls
Spin Columns for agarose beads
|
SPECIFICATIONS
|
|
|---|---|
| Configuration: | Specific Camelidae antibody linked to agarose bead |
| Particle Size: | ~90 µm when coupled to an agarose beads
~40 µm when coupled to magnetic agarose |
| Storage Buffer: | 20% EtOH |
| Part Numbers | YTA010, YTA020, YTA100, YTA200,YTA400, YTAK020, YTMA010, YTMA020, YGTMA100, YTMA200, YTA400, YTMAK020, YT250 |
| Storage and Stability: | store at 4°C; stable for one year. Do not freeze |














