Nano-Booster Fluorescence Enhancers
Elevates and Stabilizes Signals
Makes your GFP, RFP, Histone, and Vimentin really shine
Fluorescent proteins are a powerful tool to study protein localization and dynamics in living cells. However, being genetically encoded and not chemically engineered, proteins such as Green Fluorescent Protein (from jellyfish) and Red Fluorescent Protein (from mushroom coral) inherit some general shortcomings. Signal intensities of fixed samples from cells expressing a GFP- or RFP-fusions at physiological expression levels are usually very low. Also, the photostability and the quantum efficiency of these chimeric proteins are not sufficient for Super Resolution Microscopy (e.g. 3D-SIM or STED). Many cell biological methods such as HCl treatment for BrdU-detection, the EdU-Click-iT™ treatment or heat denaturation for FiSH lead to disruption of GFP or RFP signal. GFP-Boosters and RFP-Boosters are very small, binding proteins highly specific for GFP or RFP and covalently coupled to the superior fluorescent dyes from ATTO-TEC. The GFP/RFP binding domain is derived from the proprietary Alpaca-produced Nanobodies used in our popular Nanobody-Trap Affinity Reagents. Nano-Boosters provide a much stronger and more stable signal of ONLY your fusion proteins in fixed cells. Both GFP-Booster and RFP Booster are linked to different and very efficient dyes ranging from green, to red, right up to far red in emission. Choose the one that is perfect for your study. These varieties allows for many more post-growth analyses than previously thought possible for GFP, RFP, Histone, and Vimentin fusion proteins.

Above is an illustration of how Nano-Boosters can bind to fixed and permeabilized cells. The protocol is simple and the results are often unmistakable.
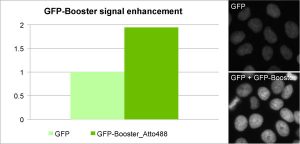
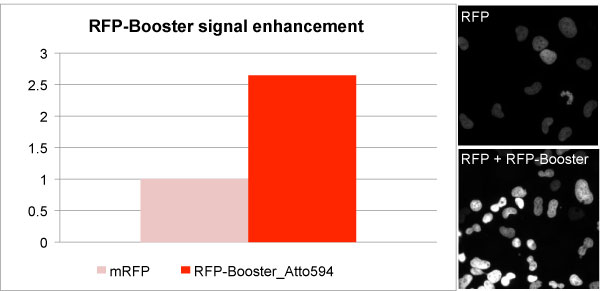
Enhancement of GFP and RFP signal with GFP-Booster (left) and RFP-Booster (right). Comparison of signal intensity was performed using a HeLa cell line stably expressing a nuclear GFP- or RFP-fusion protein before and after Nano-Booster treatment. Cells were fixed and permeabilized before treatment with Nano-Boosters.
Are Nano-Boosters right for my experiments?
In general, Nano-Boosters give similar increases in fluorescence intensity as compared with classical primary/secondary antibody techniques. Perhaps the most common issue that Nano-Boosters address is the correction of photo-bleaching of GFP- or RFP-fusions when they’re exposed to a strong excitation source. But they fix far more issues than that, just by merit of their tiny size. First, the protocol is simpler; you need only bind one miniscule molecule to your target GFP fusion. This minimizes perturbation to the structures and reduces background caused by the secondary antibody. It also shortens the protocol, saving you time. Second, Nano-Boosters can squeeze into small places so that hard-to-reach antigen sites on GFP- or RFP-fusions still bind well. Third, because Nano-Boosters are so small as compared with an antibody sandwich complex, microscopy can be of the highest resolution levels while still providing accurate localization/spatial data. Good things really do come in small packages!
Camelidae single-domain antibodies are like IgGs on steroids
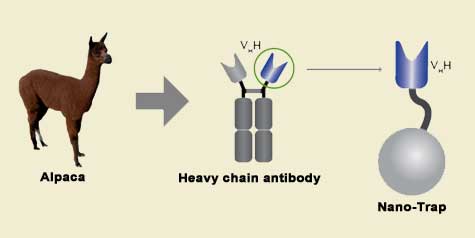
The family of animals known as Camelidae (camels, llamas, and alpacas) produce functional antibodies devoid of light chains, so-called "heavy chain" antibodies. These heavy chain antibodies recognize and bind their antigens via a single variable domain. When cleaved from their carboxy tail, these barrel-shaped structures (2x3 nm) are extraordinarily small, naturally-occurring, and intact antigen binding fragments (MW of 13 kDa). These fragments, called Nanobodies, are characterized by high specificity, affinities in the low nanomolar range, and dissociation constants in the sub-nanomolar range (typically 10- to 100-fold better than mouse IgGs). The compact size of Nanobodies makes them extremely stable at temperatures up to 70°C, and functional even in 2M NaCl or 0.5% SDS. These small and powerful antibody fragments can be used in a variety of unique applications. They will open up your research possibilities.
GFP Booster
RFP Booster
Histone Label
Vimentin Label
|
SPECIFICATIONS
|
|
|---|---|
| Part Numbers – GFP Booster | GB2AF48810, GB2AF48850, GB2AF56810, GB2AF56850, GB2AF64710, GB2AF64750, GBA488TS10, GBA488, GBA59410, GBA594, GBA647N |
| Specificity: GFP-Booster |
CFP, eGFP, AcGFP, mClover (complete list for other derivatives) |
| Protein Concentration: GFP-Booster |
0.5 – 1 mg/mL (conjugates) |
| Part Numbers – RFP Booster | RB2AF56810, RB2AF56850, RB2AF64710, RB2AF64750, RBA59410, RBA594 |
| Specificity: RFP-Booster |
mRFP, mCherry, mRFPruby, mPlum (complete list for other derivatives) |
| Protein Concentration: RFP-Booster |
0.5 – 1 mg/mL (conjugates) |
| Part Numbers – Histone-Label | tba48810, tba488100 |
| Specificity: Histone-Label |
Histone H2A-H2B heterodimers |
| Protein Concentration: Histone-Label |
0.5 – 1 mg/mL (conjugates) |
| Part Numbers – Vimentin Label | VBA48810, VBA488100 |
| Specificity: Vimentin-Label |
Vimentin-Label specifically binds to the vimentin intermediate filament protein. Validated on canine MDCK cells, rodent BHK cells and on human A549 cells upon stimulation with TGFβ. |
| Protein Concentration: Vimentin-Label |
0.3 – 0.5 mg/mL (conjugates) |
| Compatability | immunofluorescence (IF)
Histological stainings (IHC) Super Resolution Microscopy (SRM) |
| Starting Material | Cell lines and tissue samples |
| Storage Buffer | 1x PBS, preservative: 0.01% Sodium Azide |
| Storage & Stability | Store at -20°C; Stable for 6 months. Protect from light. |