RFP-Trap Affinity Reagents
Novel Antibody Fragment that binds RFP Fusion Proteins
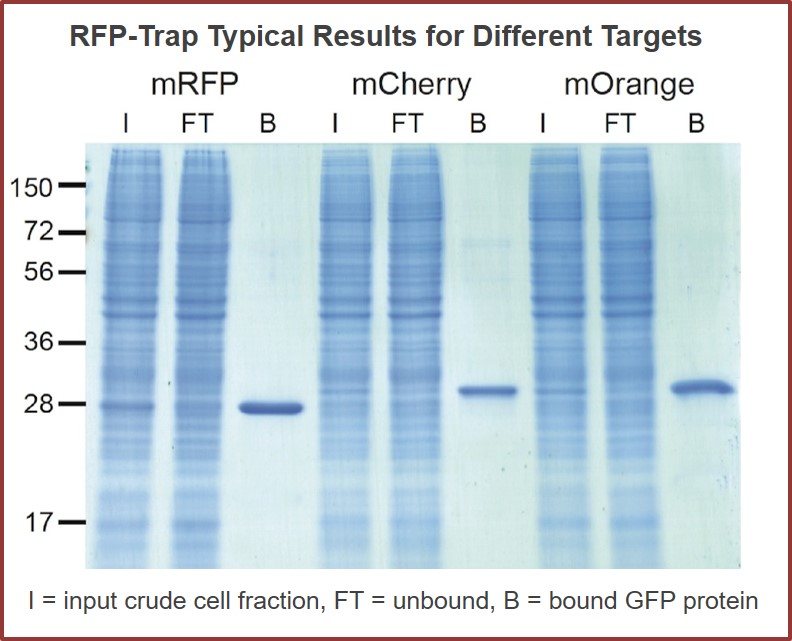
Analyze RFP fusion proteins – in a tube
These constructs usually fuse the entirety–or at least a functional domain–of a target protein to one of the many types of reporter fluorescent proteins. Those originally derived from the mushroom coral Discosoma sp. are commonly referred to as red fluorescent protein, or RFP. It’s easy to image cells using a RFP-fusion protein construct, though to get the full picture such imaging data is often combined with additional biochemical information for the protein (or protein domain) of interest. For such biochemical experiments, typically a second fusion protein is designed using a different “tag” domain that allows for purification. These additional in vitro analyses can be used to confirm functionality of the “tagged” fusion construct, as well as to pull out multi-protein complexes that may form in the cellular milieu. The lack of specific, reliable, and efficient reagents has limited the use of RFP, and related fluorescent fusion proteins, for use in both cell biology studies and direct biochemical analysis.
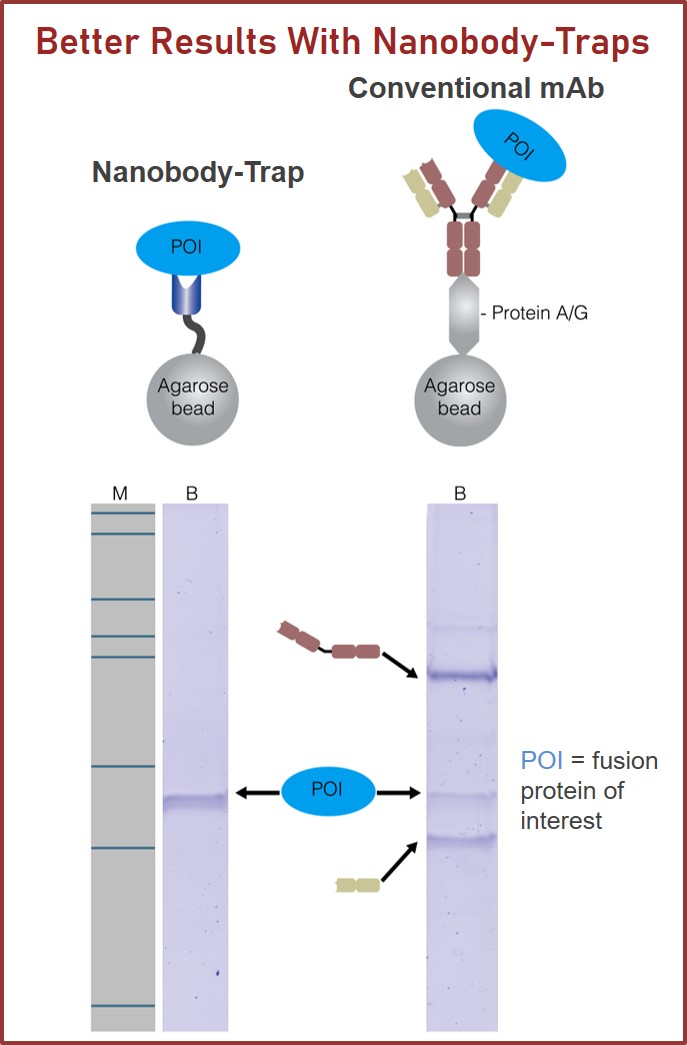
RFP-Traps utilize super-high affinity alpaca antibody fragments coupled to agarose beads or magnetic agarose beads. These “Nanobody-Traps” are perfect for immuno-precipitation, immuno-purification, and immuno-pulldown experiments with up to 10-fold better purity (and yield) than that of conventional mouse monoclonal antibodies. Compatible with a variety of source materials, Nanobody-Traps may be used with mammalian cells, tissues & organs, bacteria, yeast, and even plants. These reagents allow your RFP-fusions to be perfect candidates for immunoprecipitations, Co-IP, mass spectroscopy, enzyme activity measurements, and ChIP analysis.

Nanobody-Trap Affinity Reagents are perfect for fast, clean, and efficient one-step immuno-purification of these fluorescent fusion proteins and their interacting factors. Manufactured since 2008, and with thousands of published citations attesting to their broad use and effectiveness, we invite you to try this unique reagent for free. They’ve become a staple of cell biology research throughout Europe and North America.
What’s your lab’s favorite flavor?
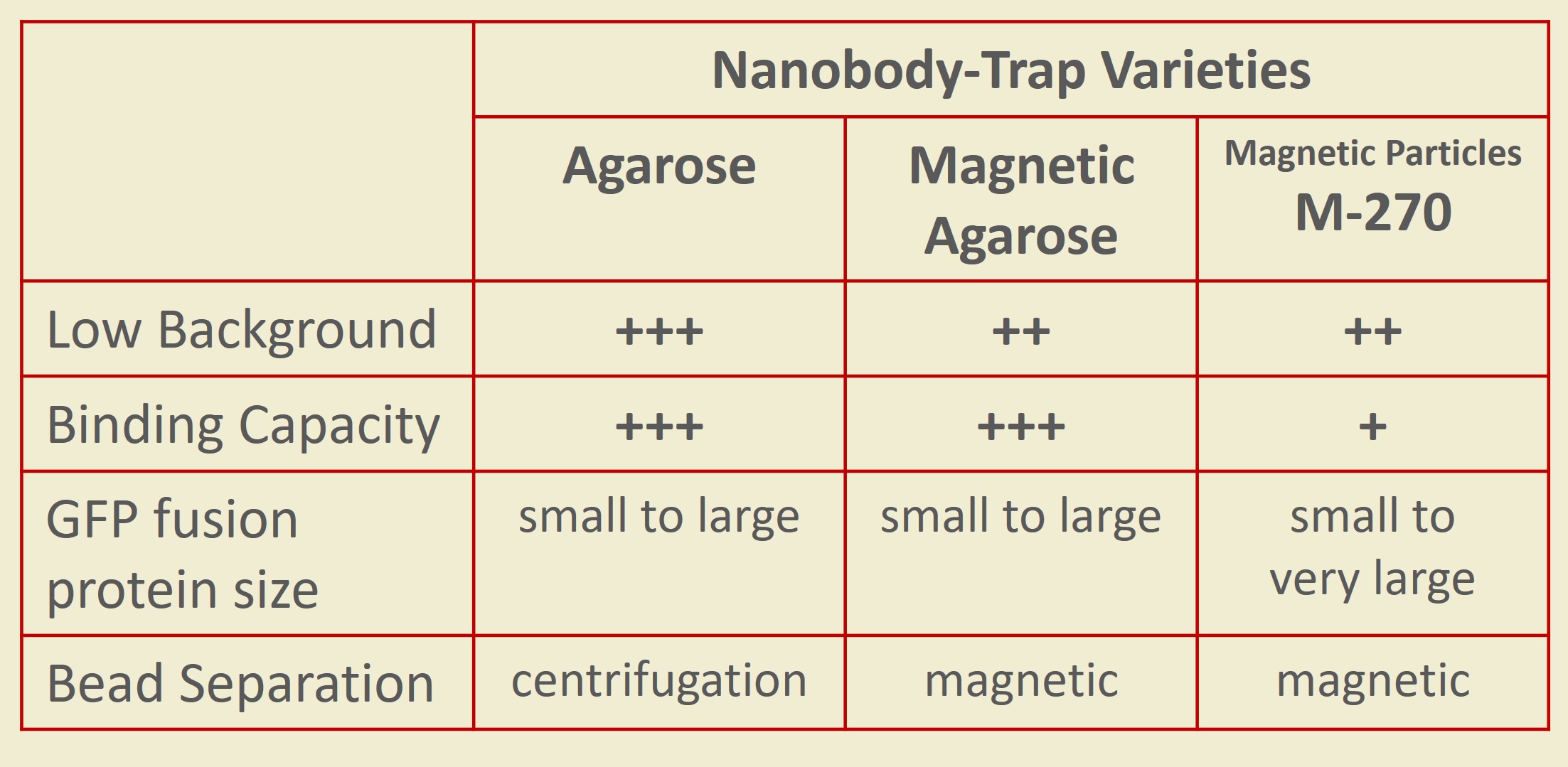
Nanobody-Traps are high quality binding proteins coupled to a monovalent matrix (agarose beads, magnetic particles) for biochemical analysis of many fusion proteins and their interacting partners. The different formats allow you to perform a multitude of experiments. The most popular forms are Nanobody-Traps linked to agarose beads. Agarose beads provide the largest binding capacity and lowest background, while still being easy to work with. If automation is important to your lab, then magnetic agarose particles are a great choice. The purified protein complexes are compatible with down-stream analyses such as mass spectroscopy.
Over 50 fluorescent proteins have been checked with Nanobody-Traps, click to check!
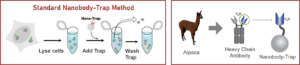
Camelidae single-domain antibodies are like IgGs on steroids
The family of animals known as Camelidae (camels, llamas, and alpacas) produce functional antibodies devoid of light chains, so-called “heavy chain” antibodies. These heavy chain antibodies recognize and bind their antigens via a single variable domain. When cleaved from their carboxy tail, these barrel-shaped structures (2×3 nm) are extraordinarily small, naturally-occurring, and intact antigen binding fragments (MW of 13 kDa). These fragments, called Nanobodies, are characterized by high specificity, affinities in the low nanomolar range, and dissociation constants in the sub-nanomolar range (typically 10- to 100-fold better than mouse IgGs). The compact size of Nanobodies makes them extremely stable at temperatures up to 70°C, and functional even in 2M NaCl or 0.5% SDS. These small and powerful antibody fragments can be used in a variety of unique applications. They will open up your research possibilities.
RFP-Traps Coupled to Agarose

RTA010 – RFP-Trap, coupled to agarose, 10 rxns, 0.25 ml
Original price was: $289.00.$284.00Current price is: $284.00. Add to cart
RTA020 – RFP-Trap, coupled to agarose, 20 rxns, 0.5 ml
Original price was: $499.00.$489.00Current price is: $489.00. Add to cartRFP-Traps Coupled to Magnetic Agarose Beads

RTMA010 – RFP-Trap, coupled to magnetic agarose beads, 10 rxns, 0.25 ml
Original price was: $289.00.$284.00Current price is: $284.00. Add to cart
RTMA020 – RFP-Trap, coupled to magnetic agarose beads, 20 rxns, 0.5 ml
Original price was: $499.00.$489.00Current price is: $489.00. Add to cartRFP-Traps Non-Coupled
Binding Controls
Spin Columns
|
SPECIFICATIONS
|
|
|---|---|
| Configuration: | |
|
RFP-Trap A |
Specific Camelidae antibody linked to agarose bead |
|
Part Numbers
|
RTA010, RTA020, RTA100, RTA200, RTA400, RTAK020 |
|
RFP-Trap MA |
Specific Camelidae antibody linked to magnetic agarose particle |
|
Part Numbers
|
RTMA010, RTMA020, RTMA100, RTMA200, RTMA400, RTMAK020 |
|
RFP-Trap |
Uncoupled and purified Camelidae antibody |
|
Part Numbers
|
RT250 |
| Specificity: | |
|
RFP-Traps |
mRFP, mCherry, mPlum, mOrange, mRFPruby, mRuby2, mKate2, PA-mCherry, TagRFP
(weak binding to DsRed1 and tdTomato) |
| Binding Capacity: | |
|
RFP-Trap A |
10µl binds 3-4µg of recombinant GFP/RFP |
|
RFP-Trap MA |
10µl binds 3-4µg of recombinant GFP/RFP |
| Particle Size: | |
|
RFP-Trap A |
~90 µm |
|
RFP-Trap MA |
~40 µm |
|
RFP-Trap |
No particle coupled |
| Storage Buffer: | |
|
RFP-Trap A |
20% EtOH |
|
RFP-Trap MA |
20% EtOH |
|
RFP-Trap |
1x PBS; Preservative: 0.01% Sodium Azide |
| Storage and Stability: | |
|
RFP-Trap A |
store at 4°C; stable for one year. Do not freeze. |
|
RFP-Trap MA |
store at 4°C; stable for one year. Do not freeze. |
|
RFP-Trap |
store at 4°C; stable for one year. Do not freeze. |











